The introduction of a PIM system into the IT circuit of a trading or manufacturing company is already the norm. But in the context of the IT framework of pharmaceutical companies, such a system has not yet become familiar, although there are already successful cases in world practice.
In this article, we'll explain how integrating PIM into a pharmaceutical company's outline can affect the complexity of maintaining product information, sales volumes, and brand perception.
Why businesses need a PIM system
The absence of a product information management system in the early stages of a company's life is common. Information about a small assortment that is sold through a couple of sales channels is easy to maintain manually.
However, as the number of SKUs increases and processes scale up, the old approaches can lead to chaos in product information. This, in turn, has a negative impact on the company's business performance.
There are often hundreds of different attributes associated with each SKU. For example, companies in the pharmaceutical industry store the following information about their products:
- the name and description of the drug;
- product variations — form of release (tablets in blisters, capsules, vials, etc.), the content of the active substance;
- indications and contraindications;
- visual attributes — photo/video of drug packages, documentation (drug registration certificate);
- marketing information (keywords, SEO elements, tags);
- sales information (prices, customer reviews, recommendations and reviews);
- localization information (multilingual copies of the description, translation into different languages);
- information about the manufacturer (manufacturer, place of production, supplier, etc.).
If all this information is stored in different sources and systems, information management becomes problematic. You'll have to make the same changes several times, spend time searching for the right tables and files, making sure that different sources do not contradict each other...
As a result, employees spend too much time updating, editing, and sending information, and critical errors occur in procurement and distribution processes.
Solving the problem of fragmented product information — PIM master system (abbreviated from English product information management).

The main function of the PIM system is to collect, store and export information about the company's products. Through integrations, it imports and exports to external resources text information, media, data for analytics, attributes, regional characteristics, prices, etc.
The pharmaceutical industry is one of the most heavily regulated, which means that any mistake here is too expensive in terms of the company's reputation and sales. A customized product information management system is an effective way to minimize these risks.
The PIM system frees up time for solving business problems
In the absence of a centralized repository of information about products and their attributes, companies often use ordinary tools: corporate file storage or, for example, Google Sheets. As a result:
- product data is stored in different places and in different formats;
- Any minor update of data (for example, drug descriptions and package photos) on all sales channels takes several hours;
- employees spend a lot of time searching for distributed information about goods, thus, less time is left for tasks that affect the company's revenue;
- There are errors in the data.
This situation is not exclusive to the pharmaceutical industry — similar cases happen in any field.
When PIM becomes the main system for storing product information, the company becomes certain what product information is stored, where and in what format.
Employees can easily find and update:
- information about the composition;
- instructions for use;
- information about side effects and other important characteristics;
- data on the version of the registration certificate.
As a result, employees spend less time searching and redistributing information and more time on increasing sales.
Reliable information on all channels reduces reputational risks and penalties
The introduction of the PIM system allows the manufacturer or distributor of drugs or equipment to maintain the uniformity and accuracy of information at all points and on all sales channels. This task became especially relevant for Russian manufacturers and pharmaceutical distributors with the adoption law to allow online trade in OTC drugs→ in 2020 and preparations prescription drug trading experiment→.
The lack of a unified product information system gives the green light to situations where the same errors and/or inaccurate data are copied by other market participants, since there is no mechanism for verifying information. For example, it is possible that several aggregators may offer customers a drug whose description contains an incorrect expiration date due to one error that was copied several times without double-checking the information.
This error simultaneously affects three indicators:
- it poses a risk to the lives and health of customers;
- it leads to a loss of reputation (which means a decrease in sales and, possibly, the termination of agreements with distributors);
- market regulators impose penalties for inaccurate information.
Another area where a manufacturer may experience problems without centralized product information management is marketing.
For example, a manufacturer starts an advertising campaign for a drug with updated packaging and a changed description of the drug for consumers. But at that time, the sales department had not yet sent photos of the new packaging to distributors, and if it did, the distributors did not have time to update them on their websites or put new packages on pharmacy displays.
This is because you have to compare data from new files from the manufacturer with information on the site and update product cards manually. The result: the description and photos of the drug in advertisements and on pharmacy websites may differ, which misleads customers and may encourage them to refuse to buy and choose an analogue from another manufacturer with whom “everything is clear”.
Thanks to the PIM system, such cases can be avoided: in addition to information from the manufacturer, when the integration is configured, data from regulatory systems can also be received. Connecting information systems to such a repository or introducing a public catalog service allows distributors to quickly receive data on all changes without human factors barriers.
Information that has a direct impact on consumers' health has been validated
It is important for consumers to have access to complete and accurate information about the drug. Characteristics such as dosage or indications may be updated/supplemented as new studies become available.
For example, in 2023 The Ministry of Health of the Russian Federation approved the inclusion of a new indication in the instructions for use of Deltiba® (delamanid) for the treatment of pulmonary tuberculosis→. Drug therapy has been recognized as officially safe not only for adults, but also for pediatric patients weighing strictly 30 to 50 kg. Accordingly, all drug descriptions on online platforms should be promptly changed in accordance with the update to ensure the accuracy of information for consumers.
After the changes come into force, they are reflected in regulatory sources:
- State Register of Medicines (abbreviated. GRLS);
- The list of vital and essential drugs (abbreviated. ZHNVLP);
- drug registration certificate (abbreviated. RU).
Due to the huge number of characteristics of medicines and dietary supplements and their untimely updates, dangerous incidents can also occur on online platforms. Suppose new studies have shown that the drug is incompatible with certain other drugs (which was not previously known). To avoid negative consequences, it is important to quickly communicate these changes to all distributors and make adjustments to product characteristics. If we are talking about a large pharmaceutical manufacturer with a wide distribution network, it is long and difficult to do it manually.
Once again, the PIM system comes to the rescue. Thanks to the synchronization of such a system with regulatory sources on the one hand and with information consumers on the other, it is easy to control the accuracy, completeness and availability of product information.
The PIM system can be synchronized with the information systems of market regulators directly through the API or ESB layer. Returning to the Deltiba® example: as soon as the indications for use of the medication change, updates will be downloaded automatically.
But even if there is no integration and the information is transferred to XLS or CSV, this is not a problem with PIM. You can generate an updated file in a couple of clicks. This helps to avoid situations associated with improper use of the drug by consumers.
Control of legal risks and compliance with regulatory requirements
In addition to monitoring changes in the regulatory system, any manufacturer needs constant synchronization with data from at least three regulatory information systems:
- GRLS;
- ZHNVLP;
- Information system for monitoring the movement of drugs (abbreviated. IS “MDLP”).
However, when trying to automate business processes that are directly related to regulatory requirements, difficulties often arise: the data format in different regulatory systems may not coincide. This is due, among other things, to the fact that a single standard for describing drugs in IT systems on the Russian pharmaceutical market continues to be formed.
The manufacturer is obliged to provide reliable data to regulatory systems, otherwise he risks a fine. However, inaccurate information can be caused not only by an error in the source, but also by errors in the chain of comparing data from different regulatory systems. The PIM system for pharmaceutical companies should take into account such cases and maintain the correct connection between SKUs from various sources, as well as correctly compare them with data in the user's system.
More efficient inventory management
Many drugs have different officially approved forms of release. At the same time, information about them in different regulatory systems can only partially coincide. For example, for RU No. LSR-001340/07 for one manufacturer (SPENCER BIO PHARMA LLC), there are three records in GRLS:
- 500 mg + 500 mg, 1 g × 1 piece, bottles, cardboard packs;
- 500 mg + 500 mg, 1 g × 5 pieces, bottles, cardboard packs;
- 500 mg + 500 mg, 1 g × 10 pieces, bottles, cardboard packs.

At the same time, there are two entries in the essential drugs register — for packs with one bottle and ten (with a different price for each format):
- 500 mg + 500 mg, bottles (1 piece), cardboard packs, the maximum price is 337.60 rubles. ;
- 500 mg + 500 mg, bottles (10 pcs.), cardboard packs, the maximum price is 3,375.96 rubles.
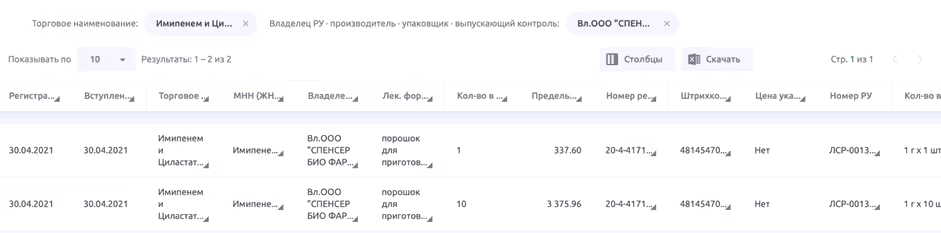
When working with registries manually, it is unlikely that there will be any problems. But when trying to automate these processes, such a seemingly uncritical difference may lead to errors in linking three GRLS entries with two entries in the essential drugs registry.
The PIM system allows you to set up an attribute model and determine a set of parameters for each item of product in order to automate and speed up the search in the information systems of market regulators. Cards for different options can be created through the “master record+guide” link — the directory will automatically collect data from the GRLS and be constantly updated. It will contain all possible packaging options that are approved for circulation.
Business processes have been automated
The PIM system not only helps to bring product descriptions in line with regulatory requirements and correctly bring them to consumers. In the system, you can create a business process for controlled work with product information.
The process allows you to regulate:
- an employee in what role (content manager, production manager or B2B sales manager) creates a product card in the system;
- who validates new information in the system;
- how data changes are reconciled;
- what happens if there is not enough information (who receives the signal, what is the sequence of actions);
- how and when to add new regulatory documents/certificates;
- how to remove goods from stock.
For each of these tasks, you can set up a clear algorithm of actions and conditions for successful and unsuccessful solutions using the functions of the PIM system. This will make it possible to more quickly detail product lines and move on to automating related business processes (for example, within supply chains).
Working with multiple markets with multilanguage support
If your company operates internationally, the PIM system will help you easily manage the translation of product information into different languages — you can quickly update information and keep it up to date in all languages.
At the same time, modern PIM systems are not limited to simply integrating an API translation tool or activating a built-in microservice. Translation functions are becoming more and more “smart”, for example, PIM systems provide translation memory.
The translation memory function is an updated database that contains a set of previously translated text segments. This is especially important in the pharmaceutical industry: if you know exactly how a phrase using specific terms should be translated, you don't run the risk of seeing incorrect language in subsequent translations.
How do I add a translation service to the PIM system? For example, Pimcore offers the choice of integrating a separate solution with the translation memory function via an API or setting up an open-source solution for translation automation. The translation memory function can work for one or more different language pairs.
Detailed analytics and business decision support
Using PIM system analytics modules, you can monitor key business parameters. For example, to verify information in product cards on online platforms with master data in the PIM system: if a retailer makes mistakes when transferring information about medicines, the system immediately signals a discrepancy. Or monitor prices for analogues from competitors and on the websites of all online retailers that distribute pharmaceutical products. Thus, you can integrate the analytical module into the Brandquad PIM system and analyze competitors' pricing policy, keywords used by customers, and calculate the share that the product occupies on electronic shelves.
Thus, drug manufacturers can more accurately manage pricing policy, as well as check whether promotions and discounts agreed with retailers are working.
The analytical module in the PIM system reduces the time to collect data for analysis and supports strategic business decisions.
Effective digital asset management
Product content (photos, descriptions and product parameters) related to medicines has a complex structure and specifics. A DAM service integrated into the PIM system will help you work with digital assets. He will take care of the following tasks.
- Storing and organizing digital assets. The DAM system provides a centralized repository for storing and organizing images, videos, audio, certificates, patents, and other files. Files can be linked to a specific product card and tagged. This greatly simplifies and speeds up the process of finding the right assets and ensures their integrity.
- Reducing the risks associated with the expiration of the “shelf life” of assets. If a patent or RU expires, the DAM system will send a notification about this. If the patent and/or RU has been updated, it will be enough to upload the updated file to PIM+ DAM — it will be transferred to all distributors through integrations. This makes it possible to significantly speed up the process of updating documents on all channels and not pausing sales during such an update.
- Access management. The DAM system allows you to manage access to digital assets. You'll be able to determine who can see, edit, or download files. To do this, you need to define roles and assign each of them a different level of access. This will increase the security and confidentiality of information.
- Process automation. The DAM system allows you to automate processes related to digital assets: changing the image size, adding watermarks or creating automatic copies. This saves time and resources on routine manual operations and improves employee efficiency.
findings
- The pharmaceutical industry is one of the most heavily regulated. Even the smallest mistake in data on a pharmaceutical company's products can lead to fines from the state and a negative impact on consumers' health.
- The PIM system centralizes product data and allows pharmaceutical company employees to quickly find and update product cards (composition, indications, instructions for use, side effects).
- The introduction of the PIM system allows pharmaceutical companies to maintain the accuracy of information at all points and on all sales channels. This makes it possible to eliminate the situation of copying errors from site to site and conduct marketing campaigns more effectively.
- By integrating the PIM system with regulatory sources and consumers of information, it is easy to ensure control over the accuracy, completeness and availability of product information. This reduces consumer health risks, reduces manual operations and ensures the consistency of information.
- The PIM system for a pharmaceutical company is a way to minimize the risk of penalties for discrepancies between information on the website and online platforms and information in regulatory systems.
- The PIM system makes it possible to create a complex attribute model and determine a set of parameters for each item of product in order to identify any package in any information system of market regulators and more effectively manage the assortment.
- Detailed centralized product information allows manufacturers to detail product lines and automate other business processes within supply chains more quickly.
- If a company operates internationally, the PIM system will help you easily manage the translation of product information into different languages, including using AI-based features that improve translation accuracy.
- A high-quality analytical module in the PIM system can reduce the time to collect data for analysis and support strategic business decisions.
- The DAM service integrated with PIM allows you to manage access to digital assets, search for them faster and more efficiently, automate some manual operations, and minimize the risks associated with the expiration date of digital assets.



.avif)




